Terms and Definitions
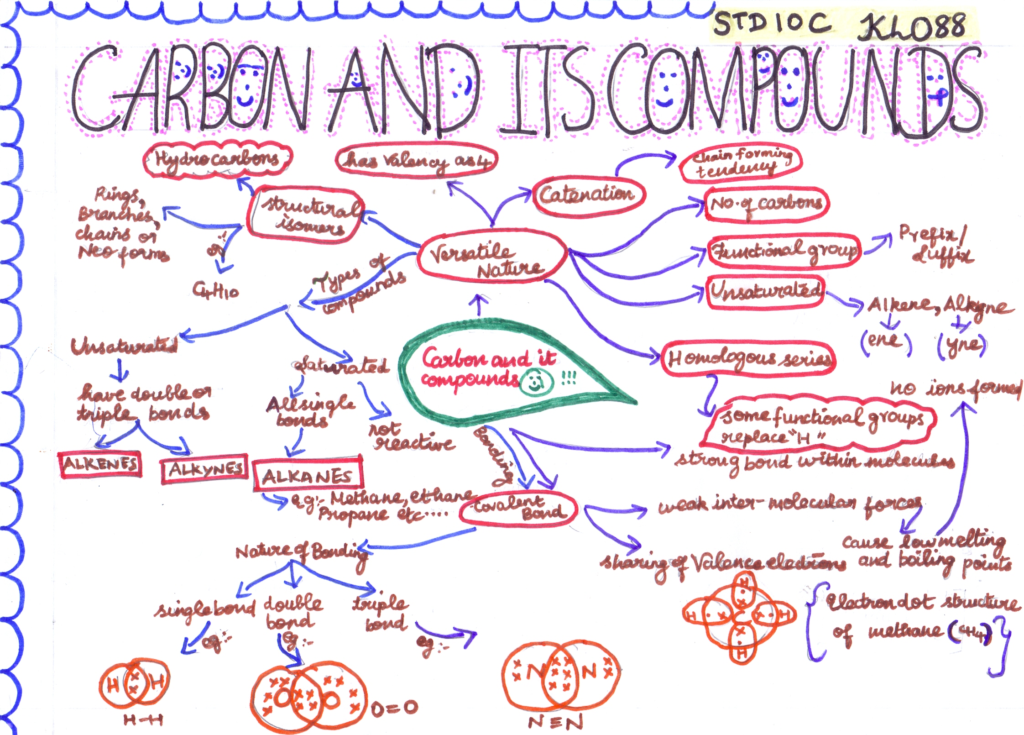
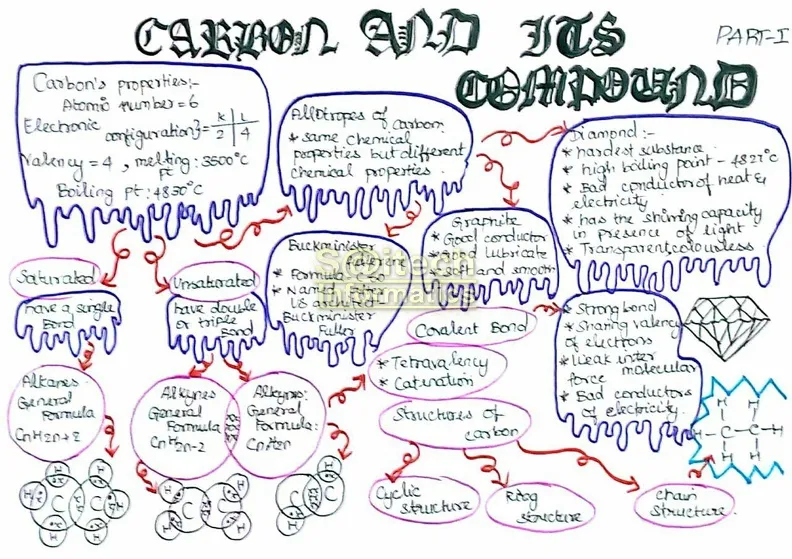
- Carbon Atom: Carbon is a versatile element that forms the basis of organic chemistry. It has six electrons, with two in the inner shell and four in the outer shell.
- Covalent Bonding: Carbon primarily forms covalent bonds with other elements, sharing electrons to achieve stability.
- Tetravalency: Carbon can form four covalent bonds, making it capable of bonding with a variety of elements, including itself.
- Hydrocarbons: Compounds composed of carbon and hydrogen are called hydrocarbons. They can be categorized into alkanes, alkenes, and alkynes based on the type of carbon-carbon bonds present.
- Alkanes: Alkanes are saturated hydrocarbons with single bonds between carbon atoms. They have the general formula CnH2n+2.
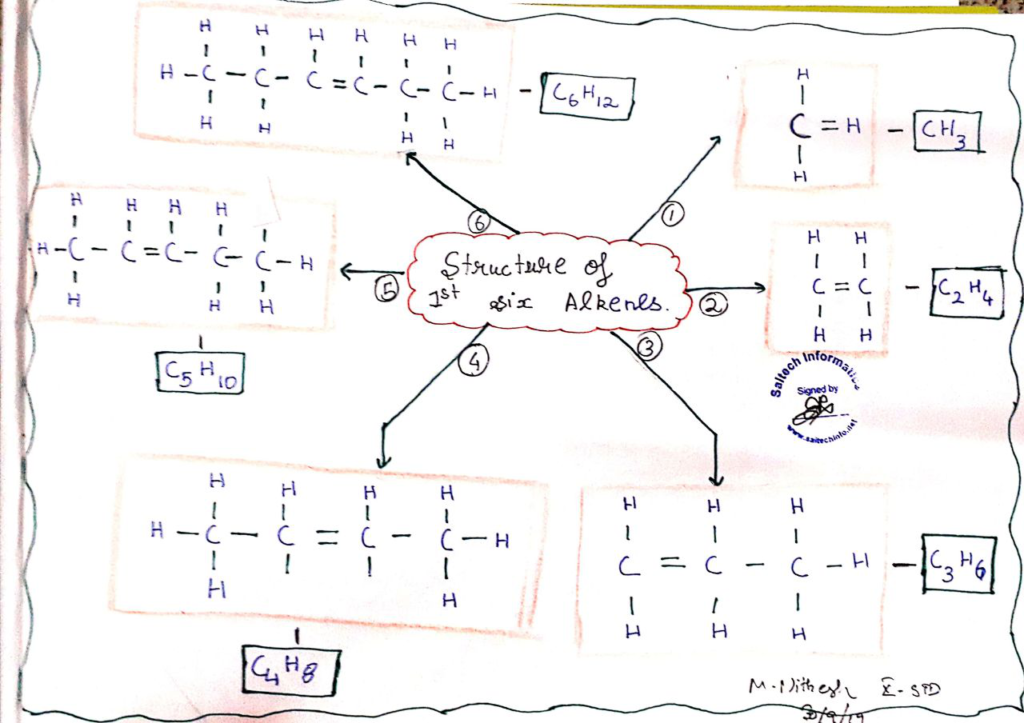
- Alkenes: Alkenes are unsaturated hydrocarbons with at least one double bond between carbon atoms. Their general formula is CnH2n.
- Alkynes: Alkynes are unsaturated hydrocarbons with at least one triple bond between carbon atoms. Their general formula is CnH2n-2.
- Isomerism: Carbon compounds can exhibit isomerism, where molecules with the same molecular formula have different structural arrangements.
- Functional Groups: Organic compounds can have various functional groups, such as alcohols, aldehydes, ketones, carboxylic acids, and more, which give them distinct properties and reactivity.
- Homologous Series: Hydrocarbons with similar structural features and a constant difference in the number of carbon and hydrogen atoms belong to a homologous series.
- Nomenclature: Organic compounds follow a systematic nomenclature system based on IUPAC rules to name them.
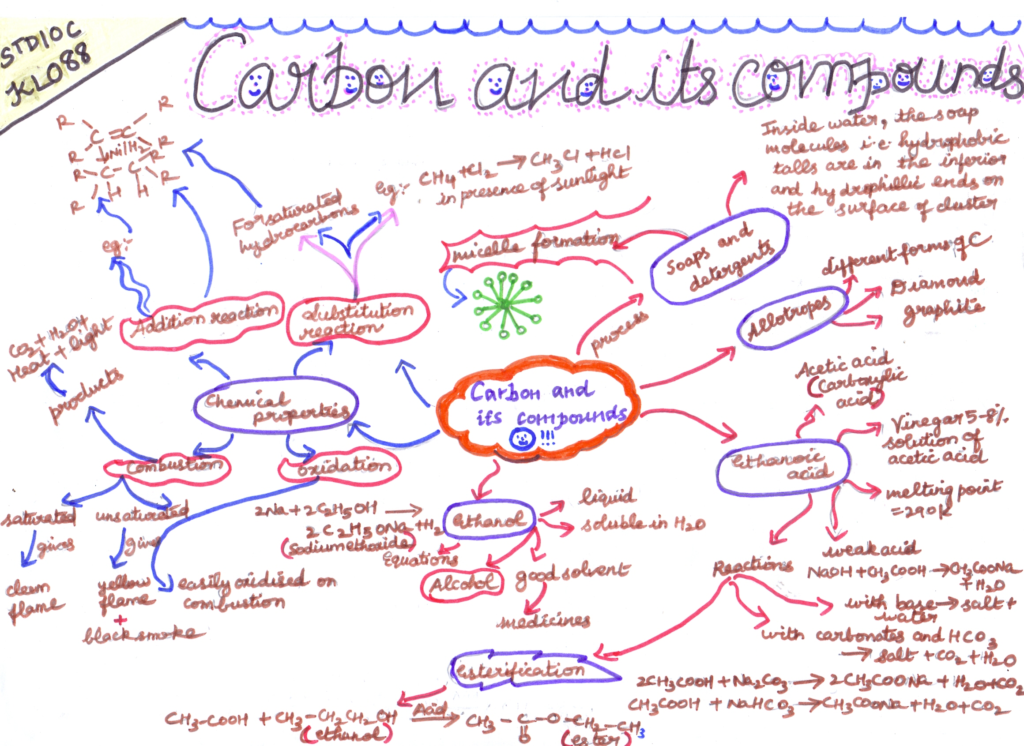
- Hydrocarbon Reactions: Hydrocarbons undergo combustion, substitution, and addition reactions, depending on their structure and the conditions.
- Saturated vs. Unsaturated: Saturated hydrocarbons (alkanes) contain only single bonds, while unsaturated hydrocarbons (alkenes and alkynes) contain double or triple bonds.
- Functional Group Reactions: Organic compounds with specific functional groups exhibit characteristic reactions, such as oxidation of alcohols and reduction of ketones.
- Importance of Carbon Compounds: Carbon compounds are vital in daily life and industry, forming the basis of polymers, fuels, drugs, and countless other products.
- Environmental Impact: The combustion of carbon compounds, such as fossil fuels, contributes to environmental issues like air pollution and climate change.
Cornell Notes in Carbon Compounds – PDF Notes
IUPAC Nomenclature Audio Drill (Listen to audio and write the molecular formula with IUPAC Name).
IUPAC Naming in Excel Sheet
Alkanes and Alkenes – Concept Video
ATOQ SET-1
- What is the IUPAC name for CH3-CH2-CH2-CH3?
a) Methane
b) Ethane
c) Butane
d) Propane - How many carbon atoms are there in pentane?
a) 2
b) 3
c) 4
d) 5 - What is the prefix for six carbon atoms in an alkane’s name?
a) Hex-
b) Penta-
c) Hept-
d) Oct- - Which of the following represents a straight-chain alkane?
a) CH3-CH2-CH2-CH3
b) CH3-CH-CH3
c) CH3-CH2-CH2-CH2-CH2-CH2-CH3
d) CH3-CH2-CH2 - What is the molecular formula of butene?
a) C3H8
b) C4H10
c) C4H8
d) C5H12 - Which carbon atom in a hydrocarbon molecule can form the maximum number of bonds?
a) Carbon-1
b) Carbon-2
c) Carbon-3
d) Carbon-4 - How many hydrogen atoms are there in a molecule of propane (C3H8)?
a) 2
b) 3
c) 6
d) 8 - What is the general formula for alkanes?
a) CnH2n+1
b) CnH2n
c) CnHn
d) CnHn-1 - Which of the following is NOT a hydrocarbon?
a) Methane
b) Ethene
c) Methanol
d) Propane - How many valence electrons does carbon have?
a) 2
b) 4
c) 6
d) 8 - What is the hybridization of carbon in methane (CH4)?
a) sp
b) sp2
c) sp3
d) sp4 - Which type of bond is formed between carbon atoms in an alkane?
a) Ionic bond
b) Covalent bond
c) Metallic bond
d) Hydrogen bond - What is the shape of a methane (CH4) molecule?
a) Linear
b) Tetrahedral
c) Trigonal planar
d) Octahedral - How many sigma bonds are there in a molecule of ethene (C2H4)?
a) 1
b) 2
c) 3
d) 4 - Which of the following is an unsaturated hydrocarbon?
a) Propane
b) Ethene
c) Butane
d) Pentane - What is the IUPAC name for C2H6?
a) Ethane
b) Methane
c) Propane
d) Butane - In an alkane, how many hydrogen atoms are attached to each carbon atom?
a) 0
b) 1
c) 2
d) 3 - Which of the following represents a branched-chain alkane?
a) CH3-CH2-CH3
b) CH3-CH2-CH2-CH2-CH2-CH2-CH3
c) CH3-CH2-CH(CH3)-CH2-CH3
d) CH3-CH2-CH2-CH(CH3)-CH3 - What is the main reason for the unique bonding ability of carbon?
a) High electronegativity
b) Small atomic size
c) Multiple oxidation states
d) High atomic mass - Which type of carbon-carbon bond allows for rotation around its axis?
a) Single bond
b) Double bond
c) Triple bond
d) Aromatic bond
Sketch Notes Summary

Sketch Note Summary by Pavani
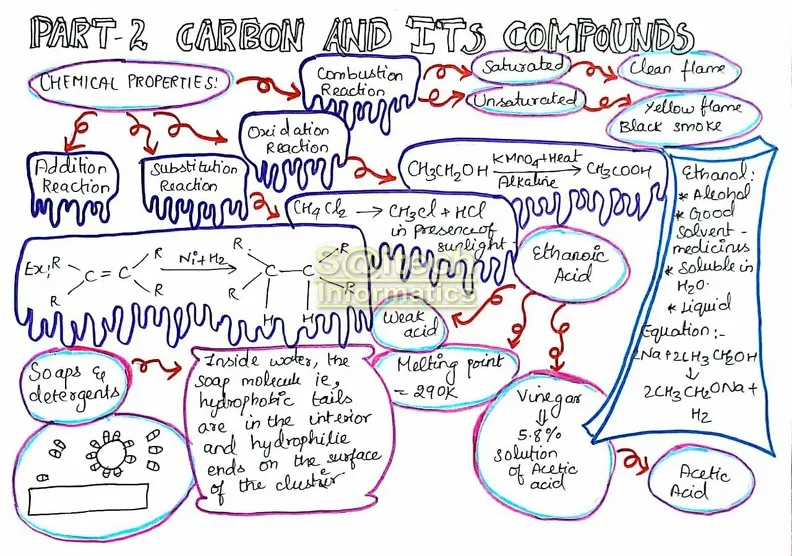
Covalent Nature of Carbon
Learn IUPAC Names using Google Spread Sheet
General formula and naming of organic compounds
IUPAC Naming and Bond Line Formula
Bond Line Formula
Commercially Important Alcohols
KEY-1
- c) Butane
- d) 5
- a) Hex-
- a) CH3-CH2-CH2-CH3
- c) C4H8
- c) Carbon-3
- d) 8
- a) CnH2n+1
- c) Methanol
- b) 4
- c) sp3
- b) Covalent bond
- b) Tetrahedral
- b) 2
- b) Ethene
- a) Ethane
- c) 2
- c) CH3-CH2-CH(CH3)-CH2-CH3
- c) Multiple oxidation states
- a) Single bond