- Dalton’s model
- J.J. Thomson’s model
- Rutherford’s model
- Bohr’s model
- de Broglie’s concept
- Heisenberg’s uncertainty principle
- Quantum mechanical model – Schrodinger Equation
- Quantum numbers
- Filling of orbitals
- Aufbau principle
- Pauli Exclusion principle
- Hund’s rule of maximum multiplicity
- Shapes of orbitals
DALTON’S ATOMIC MODEL
- Atoms are indivisible.
- a-tomio = non-divisible (Greek)
- Basic unit that makes up all matter is atom.
- Failure – atom is divisible, sub-atomic particles such as proton, electron, neutron were later found out.
J.J.THOMSON’S ATOMIC MODEL
- J.J. Thomson’s cathode ray experiment showed that atoms consist of negatively charged particles (electrons).
- Atom is a positively charged sphere in which the electrons are embedded like the seeds of watermelon.
RUTHERFORD’S ATOMIC MODEL
- Rutherford’s alpha ray scattering experiment showed that Thomson’s model was wrong.
- He bombarded a thin gold foil with a stream of fast moving alpha particles and obtained the following results.
- most of the alpha particles passed through the foil.
- some of them were slightly deflected.
- very few alpha particles were bounced back.
- Based on these observation he inferred the following proposals.
- Atom contains lot of empty space.
- Electrons are revolving around the nucleus in cricular orbits.
- Nucleus is the centre mass occuping the least space.
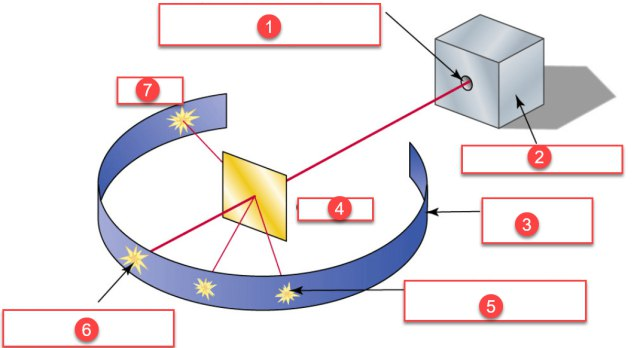
- Source of alpha particles
- Lead block shield
- Photosensitive zinc sulphide screen
- Gold foil
- Some deflected alpha particles
- Most undeviated alpha particles
- A few bounced alpha particles
- Failures – A moving charged particle continuously loose its energy in the form of radiation. The moving electron will hit the central nucleus by loosing its energy gradually and developing a spiral path. This causes a self destruction of atom.
BOHR’S ATOMIC MODEL
- The energies of electrons are quantised.
- The electron is revolving around the nucleus in circular stationary orbits.
- The electrons possess angular momentum (mvr) and that is equal to integral multiples of h/2π.
- mvr = nh/2π
- m = mass of electron
- v = velocity of electron
- n = integer
- h = Planck’s constant
- When an electron revolves in a stationary orbit, it does not loose its energy.
- However, when the electron jumps from higher energy state (E2) to lower energy state (E1), the excess energy is emitted as radiation.

- When the electron in lower energy state (E1) absorbs an energy, it jumps to higher energy state (E2).

- n = 3 (Third energy level) = M shell
- n = 2 (Second energy level) = L shell
- n = 1 (First energy level) = K shell
- Increasing energy orbits
- Nucleus
- Electron orbits
- Electron
- Emission of radiation, (E3 – E2) = hν
- Radius of nth orbit (rn)is given by

- Energy of the electron revolving in the nth orbit is given by

- Energy of the electron in the nth orbit is also given by

- Limitations of Bohr’s concept
- Applicable to species having one electon system only (H, Li2+)
- Not applicable to multi electron atoms.
- Unable to explain Zeeman effect and Stark effect.
- Zeeman effect: the splitting of spectral lines in the presence of magnetic field.
- Stark effect: the splitting of spectral line in the presence of an electric field.
- Unable to explain revovling of electrons in fixed orbit.
de Broglie Concept
- All forms of small particles of matter show wave and particle nature. (Dual nature of matter).
- According ot Planck’s quantum hypothesis, the energy is given as photon (hv), where v = frequency, h = Planck’s constant.
- According to Einstein, E = mc^2
- de Broglie combined these two equations as follows.


For a particle having mass m and moving with a velocity, v, the wavelength is given by

This equation is permitted only when the particle travels at the speed much less than that of light. The light velocity, c is replaced with matter velocity, v. Momentum, p is given by mv.

- Higher the momentum, less will be the wavelength.
- Lower the mass, higher will be the wavelength.
- Therefore, electron like particles having mass in the order of 10^-31 kg have the wavelength larger than the size of atom. Wavelength is significant.
Quantisation of Angular momentum by de Broglie concept
- The electrons revolving around the nucleus exhibit particle and wave nature.
- Circumference of the orbit of electron is given by




HEISENBERG’S UNCERTAINTY PRINCIPLE
- It is not possible to determine both position and momentum of a microscopic particle simultaneously and accurately.

Δx = uncertainty in measuring the position
Advertisements
about:blank
REPORT THIS AD
Δp = uncertainty in determining momentum
- For macroscopic objects the uncertainty is insignficant.
QUANTUM MECHANICAL MODEL (Schrodinger Equation)
- The dual nature of microscopic particles cannot be explained by classical mechanics.
- Schrodinger explained the dual nature by quantum mechanics using differential equation.
- The time independent Schrodinger equation is given by

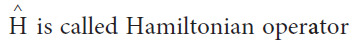



- The above equation is called time independent Schrodinger wave equation.
- The total energy, E is quantised, indicating the permitted total energy values only.
- These permitted energy values are called eigen values.
- The corresponding wave functions represent the atomic orbitals.
What are the features of the quantum mechanical model of atom?
- The energy of electrons in an atom is quantised.
- The solutions of Schrodinger wave equation gives the allowed energy levels. They are called orbitals or sub-energy levels.
- Orbital is a three dimensional space within which the probability of finding out the electron is maximum.
- The wave nature of electron in an orbital can be defined by the wave function.
- The probability density is always positive as given below.
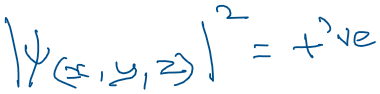
QUANTUM NUMBERS